|
Could we measure sexual interest using functional imaging?
Kirsten Jordan, Peter Fromberger, Jürgen L. Müller
Department for Forensic Psychiatry and Psychotherapy, Georg -August-University Göttingen, Germany
[Sexual Offender Treatment, Volume 10 (2015), Issue 1]
Abstract
The assessment of deviant sexual preference has a long tradition in forensic psychiatry, as it is one of the strongest predictors for sexual offence recidivism. From a clinical point of view, an objective assessment of sexual preference, i.e. independent of the patient's subjective response, is of great importance. Functional brain imaging offers one convenient approach, given the possible advantages of measuring neurofunctional processes, e.g. the possible low susceptibility to manipulations of the brain activation or the non-intrusiveness of the method. Till this day we do not have a clinical tool to assess sexual preference based on brain imaging methods. In contrast, basic functional neuroimaging research serves as a comprehensive source of knowledge about the underlying neural correlates of human sexual arousal in healthy subjects. Our review implies an overview about the most common experimental designs used in functional imaging studies measuring sexual arousal and sexual preference. Based on these studies the most recent knowledge has been summarized, showing the neural underpinnings of sexual arousal and sexual preference in healthy subjects and paraphilic patients. Results show a relatively clear network evoked by sexually arousing stimuli in healthy subjects and paraphilic patients, which can be described in the so-called four-component model of sexual arousal. Studies assessing sexual preference do not deliver such a clear picture. Despite some interesting results of several studies, till this day we do not know which brain regions respond exclusively to a sexually preferred stimulus. These results as well as methodological aspects are critically discussed in order to improve experimental designs. At last, the potential and limitations of the assessment of (deviant) sexual preference using fMRI are disputed.
Keywords: fMRI, sexual preference, sexual arousal, paraphilia, pedophilia, assessment
INTRODUCTION
At first glance, the assessment of sexual preference seems not to be a very difficult task: You can simply interview the person and in most cases a sufficient answer will be delivered. It can be seen as if a person is feeling sexually attracted by another person and/ or an object (Schmidt, Mokros, & Banse, 2013; Seto, 2012). The assessment of sexual preference becomes a very complex task in cases where respondents are unwilling and rather interested to conceal their sexual preference. Especially the forensic sector or legal settings, in which deviant sexual preference plays an important role, are confronted with that issue. On the one hand self-reports demonstrate an insufficient validity and reliability due to the tendency of participants to answer in a socially desirable manner (O'donohue, Regev, & Hagstrom, 2000). On the other hand the existence of deviant sexual preference is one of the strongest single predictors of sexual offence recidivism (Hanson & Morton-Bourgon, 2005). This fact highlights the importance of a valid and reliable assessment of deviant sexual preference, not only for risk assessment but also for diagnostic purposes, and treatment planning of paraphilia.
The penis plethysmography (PPG) seems to be one possible solution for this problem. Developed more than 60 years ago by Kurt Freund (Freund, Diamant, & Pinkava, 1958), PPG is still the gold standard in assessing deviant sexual preference in North America based on a good classification accuracy and demonstrated in a multitude of studies (Blanchard, Klassen, Dickey, Kuban, & Blak, 2001; Freund & Blanchard, 1989). But PPG is not indisputable. Primarily, because of the intrusiveness of the method the high proportion of non-responders and the inconsistent results regarding the discriminant validity and selectivity (for a recent review see (Marshall, 2014). Besides PPG, there exists a broad range of assessment methods which focus on cognitive processes for the indirect assessment of sexual interest (Thornton & Laws, 2009). The most prominent method is the viewing time (VT) approach. The VT approach is based on the finding that people show longer viewing times to stimuli that they feel sexually attracted to (in accordance with their sexual preference) than to stimuli not in accordance with their sexual preference. But even VT should be used with caution in clinical settings due to reliability and validity questions (Glasgow, 2009; Sachsenmaier & Gress, 2009). Other indirect methods demonstrated promising results as well, but mostly in fewer studies with smaller sample sizes, e.g. the implicit association task (IAT) (Brown, Gray, & Snowden, 2009), the eye tracking method (Fromberger et al., 2013; Fromberger et al., 2012), the choice-reaction-time task (CRT) (Mokros, Dombert, Osterheider, Zappala , & Santtila, 2010), the rapid serial visual presentation test (RSVP) (Beech et al., 2008) or an adaption of the emotional stroop task for sexual offenders (Smith & Waterman, 2004).
All of the above mentioned methods target on psychophysiological or cognitive mechanisms and use well- known methods to measure these processes. Interestingly, neurobiological processes and modern brain imaging techniques, like functional magnetic resonance imaging (fMRI) or positron emission tomography (PET) as a clinical assessment tool, were not in the researcher's main focus yet. Given the neurobiological hype of the last two decades and the growing availability of brain imaging techniques, this is startling, especially in the face of some possible advantages of measuring neurofunctional processes, e.g. the possible low susceptibility to manipulations of the brain activation or the non-intrusiveness of the method (Ponseti et al., 2012). Otherwise, the results of imaging studies in basic science, that is measuring sexual arousal and sexual preference in order to fathom the neurofunctional basics of the human sexuality, demonstrated that our knowledge concerning the neurofunctional patterns associated with deviant sexual preference - in contrast to normophilic sexual arousal and preference - is far from showing a clear picture.
Up to now, there is one approach using brain-imaging techniques as a clinical tool to assess deviant sexual preference. Ponseti et al. (2012) applied an automatic pattern classification method to differentiate between brain activation patterns of a group of pedophilic patients and a group of healthy subjects. They analyzed brain activation patterns in response to the presentation of explicit visual sexual stimuli. The combination of the eye tracking method and fMRI could form another approach to assess deviant sexual preference. Fromberger et al. showed that eye movements, which represent automatic attentional processes, demonstrated high diagnostic accuracy to access deviant sexual interest (Fromberger et al., 2012). Combining this method simultaneously with functional imaging could give a more detailed insight in neurofunctional mechanisms of deviant sexual interest and, apart from that, increase the diagnostic accuracy (Fromberger, 2010). As promising as those approaches seem at first, several replications using greater sample sizes will be needed in order to let fMRI become a reliable clinical assessment tool. Furthermore, knowledge about the neurobiological basis of (deviant) sexual interest seems to be essential in order to validly assess these interests.
Therefore, in the following neurobiological methods, findings and theories regarding sexual arousal and sexual preferences in healthy subjects and paraphilic patients are critically summarized and their consequences for the development of new assessment methods based on brain imaging techniques are highlighted. Finally, the potential and the limitations of the assessment of sexual preference using fMRI are discussed. Since most of our knowledge about the neurobiological basis of deviant sexual interests comes from studies with pedophiles, the review focus is mainly directed towards this special field of paraphilia.
THE MEASUREMENT OF SEXUAL AROUSAL AND SEXUAL PREFERENCE IN HEALTHY SUBJECTS
Human Sexuality - Theories
According to John Bancroft, human sexuality can be seen as a human condition which is manifested as sexual desire or appetite, associated physiological response patterns and behavior which may lead to an orgasm, or at least a pleasurable arousal, often between two people, but not infrequently by one individual alone (Bancroft, 2009).
Since more than 100 years, theoretical models exist, trying to explain human sexual behavior. In contrast to historical approaches, seeing sexuality as a primary drive, recent models discuss sexuality in the context of theories outlining emotion and motivation. Rosen and Beck postulated that the male sexual reaction is comparable with emotions which can be seen as a complex of physiological, psychological and behavioral components (Rosen & Beck, 1988). The dual-control model describes an interaction between sexual excitatory and sexual inhibitory processes at a cognitive and behavioral level (Bancroft, Graham, Janssen, & Sanders, 2009; Bancroft & Janssen, 2000).
Sexual arousal as one aspect of human sexual response has received most attention in empirical, psychological and neuroscientific research. Subjective sexual arousal can be defined as an emotional experience including the awareness of autonomic arousal, expectations of reward and motivated desire. Everaerd (1988) and Spiering and Everaerd (2007) proposed a model in which they assume an interaction between automatic and controlled cognitive processes and an incremental influence of attentional processes on subjective experience and the physiological aspects of sexual arousal.
In the context of forensic psychiatry, the assessment of sexual preference is of special interest. Sexual preference can be seen as if a person is feeling sexually attracted by another person or an object (Schmidt et al., 2013; Seto, 2012). According to current theories a sexually interesting stimulus can automatically attract attention which leads to an automatic and controlled processing of the stimulus and the development of sexual desire, arousal and, if appropriate, a full sexual response (Spiering & Everaerd, 2007).
Experimental approaches to measure sexual arousal and sexual preference
A lot of functional imaging studies have been published examining neurobiological underpinnings of sexual arousal in healthy subjects. These studies used mainly functional magnetic resonance imaging (fMRI) or positron emission tomography (PET). Despite very interesting and important results, a great methodological variability has been shown. Due to the well-known fact that different experimental methods are one important reason for different results, in the following paragraph, the most commonly used experimental designs and methods to measure sexual arousal and sexual preference will be discussed.
Experimental designs can vary due to different parameters. The most common experimental designs to measure sexual arousal are short video-sequences or photographs depicting hetero- or homosexual sexual intercourse. Subjects passively watch the stimuli. For example, the majority of the 73 studies included in a comprehensive review by Stoléru, Fonteille, Cornélis, Joyal, and Moulier (2012) used these short video sequences or photographs depicting male-female intercourse. Other studies used audio presentations of sexual scripts, photographs of nude males or females, or smelling sexual attractive pheromones. It seems to be important to exactly define what kind of stimuli will elicit what kind of response. For example, video sequences and photographs depicting explicit sexual activity will elicit different responses than photographs of nude persons in a non-explicit sexual position. Supposedly, the first kind of stimulation elicits a stronger sexual arousal than the latter one, also to be seen in brain activation.
Control conditions vary between neutral pictures, humorous pictures, sport video sequences, nonsexual male-female interactions, or photographs. The selection of an adequate control condition is one of the most important methodological aspects in functional brain imaging studies. Due to the classical subtraction approach, differences are usually calculated between the experimental and the control condition. Different control conditions lead to variable differential activation patterns and thus lead to a larger variability between the studies. This can be seen in activation differences between a sexually explicit condition (pornographic picture, intercourse) and a neutral condition like sports or humor where the differences will be greater than between sexually preferred (pornographic picture of a woman) and sexually non-preferred pictures (pornographic picture of a man). Furthermore, in a meta-analysis of Poeppl et al., they distinguished their studies according to the type of analysis of sexual arousal (Poeppl, Langguth, Laird, & Eickhoff, 2013). The analyzed brain activation caused by visual sexual stimuli compared to the control stimuli differed from brain activation patterns in association with penile erection.
Likewise, different stimulus presentation times can modulate the activity of certain brain regions, which are involved in the development of sexual arousal. For example, a fMRI-study of Sundaram et al. presented erotic video clips showing sexual interactions for 3 minutes to heterosexual men (Sundaram et al., 2010). Analyzing the first, second and third minute separately, significant increases and decreases in various brain regions have been found, which in turn, were involved in different stages of sexual arousal. For example, hemodynamic responses in the thalamus decreased from the first to the third minutes, whereas hemodynamic responses in the amygdala, the inferior frontal gyrus, the insula and mid brain structures increased. Sundaram et al. suggested that the first minute may represent an early stage of sexual arousal comprising cognitive, emotional and motivational components. The "late" stage of sexual arousal, e.g. the third minute, may illustrate the fully developed sexual arousal with higher level of genital response as well as the cognitive processing of the stimuli (Sundaram et al., 2010). Kagerer et al. also discussed that a short presentation time for about 4 sec will measure only the very early stage of sexual arousal, e.g. most likely reflecting the initial autonomic responses, hedonic feelings and attention in contrast to longer presentation times up to minutes (Kagerer et al., 2011).
Neurobiology of sexuality and the measurement of sexual arousal
Most studies, which are interested in the underlying neurobiological processes of sexuality in healthy subjects, mainly examined sexual arousal in heterosexual healthy male volunteers.
Based on the above mentioned, theoretical models and results of a functional brain imaging study, Stoléru et al. (1999) proposed a neurobiological model of sexual arousal, the so-called four-component model. Similar to the dual-control model, excitatory and inhibitory processes in the development of sexual arousal are proposed. Excitatory processes contain a cognitive, emotional, motivational and an autonomic component which are assumed to be closely coordinated. Based on 73 functional brain-imaging studies, Stoléru et al. (2012) adapted their four-component neurophenomenological model (see figure 1).
According to their model the cognitive component embodies a process through which a stimulus is perceived, categorized as a sexual incentive and quantitatively evaluated. First, sensory processes take place in the primary and secondary visual sensory lateral occipital and temporal cortices. Lateral occipital brain regions, which are not explicitly involved in the model, are proposed to be not only involved in the processing of lower level features like luminance, color or shape. The processing of the general emotional salience and the specific sexually arousing character of the stimulus are assumed to be the source of the activation of these brain regions. Thereafter, the appraisal of the sexual stimuli is considered to be the earliest one, linked with the right lateral orbitofrontal cortex (OFC) and the inferior temporal cortex. Subsequently increased attentional processes to the sexual stimuli have been associated with the activity in the inferior and superior parietal lobule (IPL, SPL). Whereas motor imagery in relation to sexual behavior should be linked with the ventral premotor cortex, the supplementary motor area (SMA), the cerebellum and the IPL. The specific hedonic quality of sexual behavior, e.g. pleasure associated with the rising arousal and the perception of bodily changes such as penile tumescence that characterize the emotional component, seems to be associated with the activity in the amygdala, in the somatosensoric cortex (SI, SII) and in the posterior insula. The thalamus, which is not included in the model but discussed in the review, is considered to be linked with the general emotional arousal that accompanies sexual arousal and at the same time the perception of erection (Stoléru et al., 2012). The motivational component comprises the processes that direct the behavior towards a sexual goal, including the perceived urge to express overt sexual behavior. Brain regions such as the anterior cingulum (ACC), the claustrum, the hypothalamus, the subtantia nigra and the ventral striatum are assumed to be associated with the motivational component. Additionally, the activation of the posterior parietal lobule might be related to its role in motor imagery processes, e.g. the imagination of overt sexual behavior. Cardiovascular, respiratory, and genital responses constitute the autonomic and endocrinological component, leading the subject to a state of physiological readiness for sexual behavior. The anterior cingulum, the anterior insula, the hypothalamus, and the putamen are structures that are associated with these processes. According to the model inhibitory processes involve (i) the inhibition and devaluation of sexual arousal and (ii) the inhibition of overt behavioral expression. The left lateral and medial OFC and the lateral temporal cortex are assumed to be involved in the former processes, while caudate nucleus and the caudal part of the ACC seem to be involved in the latter processes.
An interesting region in this context is the frontal lobe, which has been discussed by Stoléru et al. but not explicitly included in the model. Activations are mainly described in the inferior but also in the middle and superior frontal and the prefrontal gyrus. The inferior frontal regions, esp. BA 44/45 (brodman area) are known to be a part of the mirror-neuron system, a system of neurons that fire in either case: when an action is performed and a similar or identical action is passively observed, as well as in situations of imitation (Molenberghs, Cunnington, & Mattingley, 2012; Rizzolatti & Craighero, 2004). Thus, it is possible that subjects imagined sexual activity with the displayed sexually preferred person. The dorsolateral prefrontal cortex is known for its role in modulating executive cognitive functions of goal-directed behaviors, including attention, self-regulation, planning, inhibition, and control of impulsive behavior. This region has a lot of reciprocal connections with cortical and subcortical regions (Wood & Grafman, 2003). This leads to the assumption that the inferior frontal regions should be linked with cognitive and inhibitory components of sexual arousal.
Summing up, the four-component model illustrates that a lot of brain regions are in involved in the development and control of sexual arousal. Nevertheless, it has to be pointed out that none of these brain regions are specifically and exclusively associated with the processing of sexual stimuli, rather they are involved in a lot of other non-sexual functions. Additionally, not all the studies, which are included in the review of Stoléru et al. (2012), found an activation of all the brain regions of the four-component model. Furthermore, it appears that several structures which play an important role in the processing of sexual stimuli and are discussed in the review are not included in the model, e.g. the occipital cortex, the thalamus and frontal regions. One of the possible reasons could lie in the different methodological designs and control conditions used in the studies as described shortly in the former section (for details see: Jordan, Fromberger, Stolpmann, & Muller, 2011a; Stoléru et al., 2012).

 | 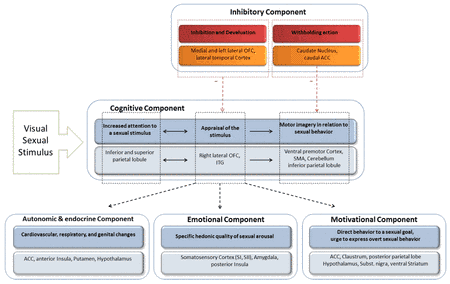
| Figure 1: The four-component model of sexual arousal. Adopted and modified from Stoléru et al. (2012) and Jordan, Fromberger, and Müller (2013) |
Recently, Poeppl et al. proposed to distinguish between the so-called psychosexual arousal and physiosexual arousal (Poeppl et al., 2013). Psychosexual arousal is defined as the core mental operations during the processing of sexual stimuli, including early automatic appraisal and attentional phenomena, which may eventually "lead to subjective experience of sexual arousal and genital response" (Janssen, Everaerd, Spiering, & Janssen, 2000; Poeppl et al., 2013). Physiosexual arousal was associated with penile tumescence and turgidity, as it seems to be the most valid indicator of male sexual arousal (Freund & Blanchard, 1989). In their meta-analysis, Poeppl et al. differentiated between brain imaging studies (i) analyzing brain activation in relation to visual sexual stimulation (e.g. psychosexual arousal), and (ii) analyzing brain activation in association with penile erection (e.g. physiosexual arousal). Most of the 20 included studies were also involved in the review by Stoléru et al. (2012) and used sexual explicit stimuli depicting female-male intercourse.
Brain networks, which showed a stronger activation during psychosexual arousal compared to physiosexual arousal, involve the inferior lateral occipital cortex, the superior and inferior parietal lobule, the caudate nucleus, the inferior frontal gyrus, the hippocampus, and the amygdala. On the contrary, the insula, parts of the opercular cortex and of the middle and anterior cingulate cortex showed stronger activation during physiosexual arousal compared to psychosexual arousal (Poeppl et al., 2013). Considering the above discussed four-component model by Stoléru and colleagues, psychosexual arousal (associated with visual sexual stimulation) can be allocated to all four components, whereas physiosexual arousal (associated with penile erection) should mainly be related to the autonomic component. Despite this crucial differentiation between psychosexual and hysiosexual arousal, the study of Poeppl et al. places emphasize on the issue of the variability between the imaging studies examining sexual arousal. According to them, the variability of results depends on the diverse ways of data analysis.
As mentioned above, from the perspective of motivational psychological theories, sexuality can also be defined in the sense of a reward behavior (Georgiadis & Kringelbach, 2012). Based on these assumptions, Georgiadis and Kringelbach described neurobiological correlates of the sexual response cycle with sexual desire, sexual arousal, plateau, orgasm and satiety or post-orgasmic refractory period (see also (Kaplan, 1979; Masters & Johnson, 1966)). Brain regions associated with sexual desire and sexual arousal are similar to those already described in the four-component model. According to the few studies examining sexual orgasm and refractory period, orgasm is characterized as a decrease of activation in brain regions known to be involved in sexual arousal, esp. in middle anterior and medial orbitofrontal regions. That decrease of activation can be seen as a disinhibition which is necessary for the initiation of an orgasm. According to the neurobiology of the reward cycle these regions may represent the emotional pleasure of reward (Georgiadis & Kringelbach, 2012). The activation pattern associated with the satiety or post-orgasmic refractory period seems to be similar to the currently discussed "default-mode" of the brain (see also (Otti et al., 2012; Raichle et al., 2001)) with activations in the ventromedial prefrontal cortex, the subgenual and pregenual anterior cingulate cortex (ACC), the anterior hypothalamus, the amygdala and the parahippocampal gyrus (Georgiadis & Kringelbach, 2012).
The measurement of sexual preference in healthy subjects
The above discussed models focused on neurobiological underpinnings of sexual arousal. Only a few functional imaging studies were interested in the neural networks associated with sexual preference, esp. with hetero- and homosexual sexual preference in healthy subjects. These studies are very important considering the challenge in the forensic psychiatry to rather differentiate between normophilic and paraphilic sexual preference instead of measuring sexual arousal.
Similar to the studies examining sexual arousal these studies presented sexually explicit stimuli (e.g. sexual activity, sexually aroused genitals), but some of them also used sexually non-explicit stimuli (e.g. nude males & females, faces). According to a study with 53 hetero- and homosexual men and women, the ventral striatum, the centromedian thalamus and the premotor cortex as well as posterior parietal areas, respond specifically to the sexually preferred stimulus when compared with a non-preferred stimulus (Ponseti et al., 2006). Comparing the hemodynamic responses to a sexually preferred stimulus (female and male sexually aroused genitals) with hemodynamic responses to a sexually non-preferred stimulus (male and female aroused genitals respectively), Ponseti et al. found a similar activation pattern in all four groups. The authors concluded that sexual core stimuli are sufficient to trigger neural responses in the mesiocentral structures of the human reward system. Interestingly, comparing both, sexually preferred and non-preferred stimuli with non-sexual control stimuli (non-sexual stimuli of the International Affective Picture System (IAPS) (Lang, Bradley, & Cuthbert, 2001), an increase in the left lateral occipital cortex, bilateral parietal areas, the ACC, the ventral striatum, the centromedian thalamus, the OFC and at a weaker statistical threshold in the amygdala, has been detected. As mentioned above only some of these regions responded specifically stronger to the sexually preferred stimulus (Ponseti et al., 2006).
Not only sexual explicit stimuli but also faces seem to evoke activation patterns, resembling the individual sexual preference (Kranz & Ishai, 2006). In a group of 40 hetero- and homosexual men and women, the thalamus and the medial orbitofrontal cortex responded most strongly to the sexually preferred face when compared to the sexually non-preferred face (Kranz & Ishai, 2006). The presentation of sexual explicit videos, depicting heterosexual or homosexual intercourse, elicited similar hemodynamic responses associated with the four components of sexual arousal in heterosexual and homosexual men while watching the film clip corresponding to their sexual orientation when compared to a sexually neutral film clip (Paul et al., 2008). Interestingly, film excerpts opposite to the sexual orientation of the subjects evoked hemodynamic responses in brain regions associated with intense (aversive) autonomic and emotional response (e.g. bilateral insula, amygdala), except in the hypothalamus. Thereby, the hypothalamus was stronger activated when hemodynamic responses to heterosexual intercourse videos in the heterosexual group were compared to the hemodynamic responses to heterosexual intercourse videos in the homosexual group and vice versa (Paul et al., 2008). This indicates that the hypothalamus seems to function as a key structure regarding sexual preference, mainly associated with the autonomic and motivational component of sexual arousal. Kagerer et al. did not find group differences between hetero- and homosexual men regarding the brain activation patterns in response to the preferred sexual explicit videos compared to neutral and aversive stimuli (Kagerer et al., 2011).
In summary, it seems that independently of the specific stimulus, subjects respond in a similar manner to their sexually preferred stimulus. The thalamus and the hypothalamus seem to be brain structures particularly sensitive and responsive to sexually preferred stimuli, regions which are connected to the autonomic and motivational aspects of sexual arousal. Other structures which have sometimes been found to specifically respond to sexually preferred stimuli are the posterior parietal areas, the orbitofrontal cortex, the premotor cortex and the ventral striatum. According to the four-component model these structures could mainly be associated with the cognitive component. Despite these interesting results we have to keep in mind that these are only a few studies with large methodological differences, e.g. regarding the type of stimulation, the specific stimuli, the control condition etc.. It is still questionable if there exist brain structures, which respond in a specific manner in dependence on the individual sexual orientation.
In the following sections we introduce the subject of paraphilia. Before discussing the studies examining sexual (deviant) interest of those patients, we describe the most recent knowledge about neurobiological models of paraphilia, esp. pedophilia.
MESUREMENT OF SEXUAL PREFERENCE IN PARAPHILIA
Neurobiological models of paraphilia
The Integrated Theory of Sexual Offending (ITSO) provides a framework to explain the onset, development, and maintenance of sexual offending.(Ward & Beech, 2006) It incorporates factors that affect brain development, like evolution, genetic variations and neurobiology, as well as ecological factors, like social and cultural environment, personal circumstances and physical environment and their impact upon neuropsychological functions. In relation to the neurobiological factors, deficits in three interlocking neuropsychological systems are proposed, the motivational and emotional system, the action selection and control system, and the perception and memory system (Pennington, 2002; Ward & Beech, 2006). The ITSO provides for the first time a theoretical framework for sexual offending considering neurofunctional factors. But, to our knowledge, the hypotheses regarding the neurobiological basis of sexual offending remain untested.
Other theories tried to describe the neurobiological basis of paraphilia based on recent findings of neuroimaging studies, most prominent for pedophilia (Jordan, Fromberger, Stolpmann, & Muller, 2011b). To date, three neurobiological theories of pedophilia have been proposed (Cantor et al., 2008). The frontal-dysexecutive theory has been put forward by Graber (Graber, Hartmann, Coffman, Huey, & Golden, 1982) and assumes that structural and functional damage of the frontal lobe might lead to behavioral disinhibition, which favors pedophilic behavior. Support for this theory comes from Burns et al. in a case study of a man who developed pedophilic behavior due to an orbitofrontal tumor (Burns & Swerdlow, 2003). Structural and functional imaging data obtained by Schiffer et al. also supports this theory (Schiffer, Krüger, et al., 2008; Schiffer, Paul, et al., 2008; Schiffer et al., 2007). The critical point in this theory is the lack of specificity: frontal disturbances and the consequent disinhibition are associated with impulsive-aggressive behavior, sexually disinhibited behavior and the loss of moral concepts (Fromberger, Stolpmann, Jordan, & Müller, 2009). The temporal-limbic theory posits that the temporal and limbic brain regions play a major role in sexual functions. Especially lesions of the temporal lobe are associated with hypersexual behavior (Kaplan & Krueger, 2010). Some functional imaging studies showed changes of activation in the limbic regions in pedophilic patients, thus also supporting this hypothesis (Walter et al., 2007; Wiebking, Witzel, Walter, Gubka, & Northoff, 2006). The dual-dysfunctional theory connects the former theories, assuming dysfunctions in temporal as well as in frontal brain areas. According to the latter theory, hypersexual behavior due to temporal deficits - together with behavioral disinhibition caused by frontal deficits - leads to pedophilic behavior. Structural imaging studies (Cantor et al., 2008; Cohen et al., 2002) as well as neuropsychological studies (Joyal, Black, & Dassylva, 2007) support this hypothesis.
Experimental methods to measure sexual preference in paraphilic patients
According to the fact that most fMRI studies examining deviant sexual preference are performed with pedophilic patients, the following sections will focus on this special case of paraphilia.
Till this day, 11 studies have been published examining sexual preference in pedophiles with new and interesting but also conflicting results (Cohen et al., 2002; Dressing et al., 2001; Habermeyer et al., 2013; Poeppl et al., 2011; Ponseti et al., 2012; Ponseti et al., 2014; Sartorius et al., 2008; Schiffer, Krüger, et al., 2008; Schiffer, Paul, et al., 2008; Walter et al., 2007; Wiebking et al., 2006). As already mentioned in the second section of this review, a lot of different experimental methods have been applied to measure sexual arousal and sexual preference in healthy subjects. The same is true for the 11 published studies examining a deviant sexual interest in pedophilic patients (for review see also:Mohnke et al. (2014); Polisois-Keating and Joyal (2013)).
Table 1 presents the methodological details for these studies. Besides the large variation of the number of subjects, ranging from one to 56, ten subjects were approximately included per group (Dressing et al., 2001; Ponseti et al., 2012). Most but not all studies defined the sexual preference with regard to gender and compared only subjects with the same sexual orientation. Typical pedophilic patients are compared to healthy controls. One study included a forensic control group (nonsexual offenders) (Poeppl et al., 2011). This is important due to the fact that a forensic control group is much more similar to pedophile patients than a healthy control group, for example regarding their intelligence, comorbidity, criminality, hospitalization etc. (Fromberger et al., 2012; Krüger & Schiffer, 2011).
Typically, one person is presented per trial, mostly nude persons or persons clothed in swimsuits in sexually non-explicit positions, also due to ethical aspects. One common commercially available stimulus set is the Not-Real-People picture set, which does not display images of real people and meets contemporary legal and ethical requirements. The pictures are not pornographic, depicting solely non-explicit sexual poses or sexual activities in different Tanner-stages (Laws & Gress, 2004; Mokros et al., 2011; Pacific Psychological Assessment Corporation, 2004; Tanner, 1973). The use of non-explicit sexual stimuli is in contrast to the above-discussed studies of healthy subjects, examining sexual arousal by presenting sexually explicit videos or photographs depicting sexual interaction. These differences in stimulation paradigms have to be considered when comparing brain activation patterns between the studies in pedophiles and the studies of sexual arousal in healthy subjects. Interestingly, in one study only faces of adults and children were applied (Ponseti et al., 2014).
The control stimuli are of great importance in brain imaging studies. The hemodynamic responses to these stimuli have normally to be compared with the hemodynamic responses to the experimental stimuli in order to extract only those regions linked with sexual interest or sexual arousal. As it can be seen from table 1, these control stimuli varied between studies, often due to different functions of the control condition. Some studies, for example, wanted to control the lower level features of the experimental stimuli, e.g. the color, shape, contrast etc. Therefore, simple abstract stimuli were used (e.g. (Dressing et al., 2001; Poeppl et al., 2011)). Other researchers were mainly interested in the differences between the processing of erotic and emotional stimuli (Walter et al., 2007; Wiebking et al., 2006). Therefore, the latter one served as a control stimulus. Some studies additionally compared the hemodynamic differences between preferred and non-preferred stimuli in order to distinguish the specific pattern of sexual preference (e.g. (Habermeyer et al., 2013; Poeppl et al., 2011; Ponseti et al., 2014; Schiffer, Paul, et al., 2008)).
As shown in table 1, presentation times for stimuli varied between 750 ms and 30 min. As already discussed in the second section different presentation times can lead to different stages of development of sexual arousal and thus to different activation patterns which in turn are associated with different stages of the processing of sexual stimuli.
Most studies used passive paradigms, e.g. the passive viewing of sexual stimuli, and ask the subjects to let the arousal occur. Some researchers ask the subjects to press a button if the stimulus appears in order to control subject's vigilance in the scanner. But a passive task has some disadvantages, e.g. it cannot be controlled if subjects really watch the stimuli and let the arousal occur, and they are susceptible for manipulations. For this reason, two studies chose active tasks in order to capture the attention of the subjects while presenting the visual sexual stimuli (Poeppl et al., 2011; Sartorius et al., 2008).
Despite interesting and new results these methodological differences might be one reason for the great variability regarding the results of these studies. A detailed overview for seven of the ten studies with a discussion of their potential and especially their methodological was published by Fromberger, Krippl, Stolpmann, and Müller (2007) and Fromberger et al. (2009). In the following section, the results of these ten studies will be explained in more detail. Considering the above mentioned methodological variability we mainly compare studies with similar designs or contrasts, or point out the differences between these studies.
| Table 1: Brain imaging studies examining pedophilic interest
– Experimental designs |
| Study |
Method |
Experimental
Group (EG)
Sexual Orientation |
Control group
(CG)
Sexual
Orientation |
Modality |
Stimuli
E: Experimental condition
C: Control condition |
Presentation
time
(one stimulus) |
Experimental
Design |
| Dressing et al. 2001 |
fMRI |
Pedophile
(n=1)
Homosexual |
Healthy
(n=2)
Heterosexual |
Visual
Block-design |
E: One Person in underwear/swimsuits
(common mail order catalog): Boy, woman
C: Abstract picture with similar color intensity and complexity |
19,2 sec |
Passive viewing |
| Cohen et al. 2002 |
PET |
Pedophiles (n=7)
Heterosexual |
Healthy
(n=7)
Heterosexual |
Auditive
Block-design |
E: Sexual script describing sexual
interaction: Girl-man, woman –man
C: Non-sexual words |
30 min |
Passive listening |
| Wiebking et al. 2006 |
fMRI |
Pedophiles
(n=13)
n/a |
Healthy
(n=14)
n/a |
Visual
Block-design |
E: One adult person – erotic condition
(IAPS)
C: One adult person - emotional, neutral condition (IAPS) |
n/a |
Passive viewing |
| Walter et al. 2007 |
fMRI |
Pedophiles
(n=13)
n/a |
Healthy
(n=14)
n/a |
Visual
Block-design |
E: One adult person – erotic position
(IAPS)
C: One adult person - emotional, neutral position (IAPS) |
5 sec |
Passive viewing
(button press) |
| Sartorius et al. 2008 |
fMRI |
Pedophiles
(n=10)
Homosexual |
Healthy
(n=10)
Heterosexual |
Visual
Block-design |
E: One person in swimsuits (common
mail order catalog): Girl, boy, woman, man
C: Geometrical figures (target: colored circles, non-target: colored squares) |
1 sec |
Passive viewing
(button press) & active task
Oddball-paradigm |
| Schiffer et al. 2008 |
fMRI |
Pedophiles
(n=8)
Heterosexual |
Healthy
(n=12)
Heterosexual |
Visual
Block-design |
E: One nude Person (EG: internet,
mail order art catalogues, CG: IAPS): Girl, woman
C: dressed Persons (EG: internet, mail order art catalogues, CG: IAPS):
Girl, woman |
38,5 sec |
Passive viewing
|
| Schiffer et al. 2008 |
fMRI |
Pedophiles
(n=11)
Homosexual |
Healthy
(n=12)
Homosexual |
Visual
Block-design |
E: One nude Person (EG: internet,
mail order art catalogues, CG: IAPS): Boy, man
C: dressed Persons (EG: internet, mail order art catalogues, CG: IAPS):
Boy, man |
38,5 sec |
Passive viewing |
|
Poeppl et al. 2011
|
fMRI |
Pedophiles
(n=9):
Heterosexual (n=2)
Homosexual
(n=7) |
Nonsexual offenders (n=11)
Heterosexual |
Visual
Block-design |
E: One nude Person (NRP-set)
Prepubescent, pubescent, adult (male & female)
C: One scrambled NRP-picture with similar color intensity and complexity |
4 sec |
Active task:
Choice Reaction Time Task
(CRTT) |
| Ponseti et al. 2012 |
fMRI |
Pedophiles
(n=24):
Heterosexual (n=11)
Homosexual
(n=13) |
Healthy
(n=32):
Heterosexual (n=18)
Homosexual (n=14) |
Visual
Event-related-
design |
E: One nude person or genital only:
Girl, boy, woman, man
C: nonsexual picture (IAPS) |
1 sec |
Passive viewing & active task
Oddball-stimulus |
| Habermeyer et al. 2013 |
fMRI |
Pedophiles
(N=8)
Heterosexual |
Healthy
(n=8)
Heterosexual |
Visual
Event-related design |
E: One Person in erotic condition:
Girl, boy, woman, man
C: One neutral object |
750 ms |
Passive viewing |
| Ponseti et al. 2014 |
fMRI |
Pedophiles
(n=24):
Heterosexual (n=11)
Homosexual
(n=13) |
Healthy
(n=32):
Heterosexual (n=18)
Homosexual (n=14) |
Visual
Event-related-
design |
E: Faces: Girl, boy, woman, man
C: nonsexual high-arousal picture (IAPS) |
1 sec |
Passive viewing & active task |
Notes: Only men were included in all studies Abbreviations:
EG: Experimental group, CG: Control group, SO: Sexual orientation, E: Experimental
condition, C: Control condition,
n/a – not applicable
IAPS-set: International affective picture system (Lang et al. 2001)
NRP-set: Not Real People Stimuli set for the assessment of sexual interest
(Pacific Psychological Assessment Corporation, 2004) |
Measuring sexual preference in paraphilic patients - current results of fMRI studies
The results of the 11 functional imaging studies examining sexual interest in pedophilic subjects are summarized in table 2, 3 and 4. The activated brain regions for the different contrasts are arranged according to the four-component model of sexual arousal. Considering the fact that a lot of studies reported more activated regions than those associated with the four-component model, these additional brain areas are also listed in the tables.
The first interesting question is if pedophilic subjects exhibit similar or different brain activation patterns as healthy subjects, when viewing their preferred sexual stimulus compared to a neutral, non-sexual stimulus (see table 2). Six out of the ten studies computed that kind of contrast. Five out of these six studies have found hemodynamic responses in brain regions associated with at least three out of the four excitatory components for sexual arousal, as well as in brain areas associated with the inhibitory component. Additionally, activations were mainly found in the frontal lobe, thalamus, hippocampus and occipital regions. Interestingly, Stoléru et al. (2012) discussed most of these brain regions in their review, according to the finding that a lot of studies with healthy subjects report activations in those regions as well. But they did not include these brain regions into the four-component model. Additionally, the hippocampal/parahippocampal regions, parts of the limbic system, are described to be activated in two studies. The processing of emotional aspects but also memory processes might be associated with these activations.
Thus, despite some inconsistencies we conclude that the four-component model of sexual arousal and the additionally described brain regions proposed on basis of studies with healthy subjects may also be valid for pedophilic subjects when viewing their preferred stimuli. It has to be pointed out, that not all studies reported activations in each of the four components. One possible cause for the difference in activations might lie in the usage of the type of stimuli. Studies with healthy subjects used explicit sexual stimuli, whereas studies with pedophiles used non-explicit sexual stimuli.
Table 2: Results of the brain
imaging studies examining pedophilic interest for the comparison of the
sexual preferred stimuli and the neutral stimuli in the pedophile subjects.
The activated brain regions are arranged according to the four-component
model by Stoleru et al. 2012 |
| |
Four-component model |
Excitatory
components |
Inhibitory component |
Additional brain regions
not assigned to the four-component model |
| |
Component |
Cognitive |
Emotional |
Motivational |
Autonomic |
|
|
| |
Brain Region |
Attention: IPL, SPL
Appraisal: right lateral OFC, ITG
Motor imagery: vPM, SMA, CB, IPL |
Somatosensory cortex (SI, SII)
Amygdala,
Posterior Insula |
ACC, claustrum, posterior parietal cortex, hypothalamus,
substantia nigra, ventral striatum |
ACC, anterior Insula, putamen, hypothalamus |
Inhibition and devaluation: medial and left lateral
OFC, lat. Temporal Cortex
Inhibition of motor reaction: caudate nucleus, cACC |
|
| Study |
Comparison |
|
| Dressing et al. 2001 |
Boy vs. neutral |
Right OFC ,
Fusiform gyrus (ITG) |
|
ACC
(basal ganglia) |
ACC |
|
Occipital cortex,
brain stem
r. prefrontal cortex |
| Cohen et al. 2002 |
Girl |
ITG |
|
Cingulate gyrus |
Cingulate gyrus |
Medial OFC, caudate ncl.
Inferior temporal cortex |
Thalamus
ventral sup. frontal gyrus |
| Wiebking et al. 2006 |
No such contrast |
|
|
|
|
|
|
| Walter et al. 2007 |
No such contrast |
|
|
|
|
|
|
| Sartorius et al. 2008 |
Children vs. neutral |
|
Amygdala |
|
|
|
|
| Schiffer et al. 2008 |
Nude girl vs. dressed girl |
Precentral gyrus, fusiform gyrus, ITG, IPL, SPL |
Insula, Amygdala, |
ACC, substantia nigra, SPL |
ACC, Insula |
Caudate body, Middle temporal gyrus |
Frontal lobe (VLPFC, DLPFC, FPPFC), Thalamus, Hippocampus,
parahippocampal gyrus, middle, inferior occipital gyrus, Precuneus |
| Schiffer et al. 2008 |
Nude boy vs. dressed boy |
Fusiform gyrus, ITG, SPL |
Postcentral gyrus |
ACC, substantia nigra, SPL |
ACC, putamen |
Caudate head, middle, superior temporal gyrus |
inferior, middle superior frontal gyrus,
globus
pallidus,
Precuneus,
middle occipital gyrus |
| Poeppl et al. 2011 |
Children vs. neutral |
ITG/fusiform gyrus,
Precentral gyrus, SPL |
Hippocampus-amygdala complex,
postcentral gyrus |
SPL |
|
Superior temporal gyrus |
Middle frontal gyrus
Posterior cingulate gyrus
Calcarine gyrus
Parahippocampal gyrus/hippocampus
Middle occipital gyrus |
| Ponseti et al. 2012 |
No such contrast |
|
|
|
|
|
|
| Habermeyer et al. 2013 |
No such contrast |
|
|
|
|
|
|
| Ponseti et al. 2014 |
No such contrast |
|
|
|
|
|
|
Abbreviations:
AM: Amygdala, ACC: anterior cingulate cortex, CB: Cerebellum, IPL: inferior
parietal lobule, OFC: orbitofrontal cortex, SMA: supplementary motor area,
SPL: superior parietal lobule, TC: temporal cortex, vPM: ventral premotor
cortex, VLPFC: ventral lateral prefrontal cortex, DLPFC, dorsal lateral
prefrontal cortex, FPPFC: frontopolar prefrontal cortex |
The second question is often addressed as if there were differences between hemodynamic responses of pedophiles and healthy subjects while viewing sexual stimuli. The differentiation between a normophilic and pedophilic preference is probably the most interesting question in regard to the clinical application of fMRI as an assessment tool for deviant sexual preference. Table 3 shows selected results from the 11 studies, presenting different comparisons between both groups. These group comparisons comprise the analysis of child stimuli, differences between child and adult stimuli and differences between preferred and non-preferred stimuli. Despite this large variability of the analyzed comparisons one can conclude that pedophile subjects exhibit stronger hemodynamic responses to child stimuli than healthy subjects in various brain regions associated with sexual arousal. These stronger hemodynamic responses affect the fusiform gyrus, parietal areas, the amygdala, insula and the ACC, but also prefrontal, hippocampal and thalamic activations. Some studies pointed out that pedophiles showed stronger activations than healthy subjects, when watching their preferred stimulus (children in pedophiles, adults in healthy subjects) (Poeppl et al., 2011; Ponseti et al., 2012). The involved brain regions embody all four excitatory components of sexual arousal, as well as memory and control functions. Only two studies consider the inhibitory components (Poeppl et al., 2011; Ponseti et al., 2012). Four out of the ten studies reported the vice versa comparison where control subjects showed stronger hemodynamic responses to adult stimuli than pedophiles. But the results are inconsistent, varying from no group differences (Sartorius et al., 2008), small and focal subcortical stronger activations (Poeppl et al., 2011; Ponseti et al., 2012) to widespread stronger activations in healthy subjects compared to pedophiles when viewing adult stimuli (Schiffer, Krüger, et al., 2008; Schiffer, Paul, et al., 2008). The variable stimulus presentation time, varying from 1 to 35 sec (see table 1), could form one possible reason for these inconsistent results. Thus, to this day, it is not clear, if pedophiles show generally stronger brain activation to their preferred sexual stimuli (child) in relation to the responses of healthy subjects to their preferred stimuli (adult).
The important question from a clinical point of view is whether there is an adequate method to differentiate between normophilic and pedophilic subjects or not. In our opinion the comparison between two groups according to the question whether or not they show stronger activation to child vs. adult stimuli (or vice versa), could form an adequate method. Sartorius et al. (2008) and Ponseti et al. (2012) reported the outcomes of these comparisons, but with different results. In the study of Sartorius et al. (2008), the amygdala was the only region which was stronger activated in the pedophilic group than in the control group, whereas Ponseti et al. (2012) found a widespread stronger activation in the pedophilic group compared to the healthy subjects. The latter authors provided a new classification approach to differentiate between the two groups. This approach will be discussed in the next section.
Table 3: Results of the brain
imaging studies examining pedophilic interest for the comparison between
pedophile subjects and a control group. The specific contrast per study
is given. The activated brain regions are arranged according to the four-component
model by Stoleru et al. 2012 |
| |
Four-componet
model |
Excitatory
components |
Inhibitory
component |
Additional
brain regions not assigned to the four-component model |
| |
Component |
Cognitive |
Emotional |
Motivational |
Autonomic |
|
|
| |
Brain Region |
Attention: IPL, SPL
Appraisal: right lateral OFC, ITG
Motor imagery: vPM, SMA, CB, IPL |
Somatosensory cortex (SI, SII)
Amygdala,
Posterior Insula |
ACC, claustrum, posterior parietal
cortex, hypothalamus, substantia nigra, ventral striatum |
ACC, anterior Insula, putamen, hypothalamus |
Inhibition and devaluation: medial
and left lateral OFC, lat. Temporal Cortex,
Inhibition of motor reaction: caudate nucleus, cACC |
|
| Study |
comparison |
|
| Dressing et al. 2001 |
No such contrast |
|
|
|
|
|
|
| Cohen et al. 2002 |
EG > CG for: girl |
n.s. |
n.s. |
n.s. |
n.s. |
n.s. |
|
| Wiebking et al. 2006
|
EG < CG: Erotic vs. emotional * |
Lateral parietal cortex |
Insula |
Lateral parietal cortex |
Hypothalamus, Insula |
|
Periaqueductal grey |
| Walter et al. 2007 |
EG < CG: Erotic vs. emotional * |
Lateral parietal cortex |
Insula |
Lateral parietal cortex |
Hypothalamus, Insula |
|
Dorsolateral prefrontal cortex,
occipital cortex |
| Sartorius et al. 2008 |
EG > CG for:
Child > adult |
|
Amydala |
|
|
|
|
| Schiffer et al.
2008
|
EG > CG for: girl
| Fusiform gyrus, |
|
|
|
|
Hippocampus, thalamus, pulvinar |
| EG > CG for: preferred > neutral |
|
|
|
|
|
frontal lobe: DLPFC |
| Schiffer et al.
2008
|
EG > CG for: boy** |
|
|
ACC |
ACC |
|
Ventromedial prefrontal cortex |
| EG > CG for:
Preferred > neutral** |
Fusiform gyrus |
|
|
|
|
DLPFC |
| Poeppl et al.
2011
|
EG > CG for:
Children > neutral |
Cerebellum |
Medial temporal lobe (insula) |
|
|
Middle temporal gyrus |
Posterior cingulate gyrus,
thalamus,
medial frontal lobe, hippocampus |
| EG > CG for:
Preferred > neutral |
IPL |
Postcentral gyrus,
Insula |
Anterior midcingulate gyrus,
IPL |
Anterior midcingulate gyrus,
Insula |
Middle temporal gyrus |
|
| Ponseti et al.
2012
|
EG > CG for: Boy > man |
Cerebellum,
fusiform gyrus, ITG
Angular gyrus |
|
|
|
|
Lingual gyrus,
Anterior thalamus,
Hippocampus,
Occipital lobe |
| EG > CG for: Girl > woman |
SPL, ITG, fusiform gyrus, cerebellum |
Amygdala, Insula |
Cingulate gyrus |
Cingulate gyrus, Insula |
Caudate ncl. |
Occipital lobe, inferior frontal gyrus, thalamus |
| Habermeyer et al. 2013 |
EG > CG for: Girl > woman |
right lat. OFC |
|
|
|
|
|
| Ponseti et al. 2014 |
No such contrast |
|
|
|
|
|
|
Abbreviations:
AM: Amygdala, ACC: anterior cingulate cortex, CB: Cerebellum, IPL: inferior
parietal lobule, OFC: orbitofrontal cortex, SMA: supplementary motor area,
SPL: superior parietal lobule, TC: temporal cortex, vPM: ventral premotor
cortex, VLPFC: ventral lateral prefrontal cortex, DLPFC, dorsal lateral
prefrontal cortex, FPPFC: frontopolar prefrontal cortex
CG: Control group, EG: Experimental group
* Wiebking et al. 2006, Walter et al. 2006 – analyzed decreased activation
patterns in pedophilic patients (EG) vs. healthy controls (CG) for the contrast:
erotic pictures vs. emotional pictures
** Note by the authors: comparisons need to be carefully interpreted because
the control subjects generally showed weaker activations |
Another important question regarding the measurement of sexual preference in pedophilic subjects is if they respond differentially to their preferred sexual stimulus compared to their non-preferred sexual stimulus. From a scientific point of view, this comparison allows further exploration of the neurofunctional underpinnings of a pedophilic preference. From a more clinical point of view, this contrast could allow the differentiation between the exclusive type of pedophilia and the non-exclusive type. Some imaging studies examined this question by analyzing the hemodynamic responses to children compared to adults within the pedophiles (see table 4). As can be seen from table 4 the results of the studies show a great variability regarding the reported brain regions. Taken these results together, a stronger activation to children compared to adults has been found in the OFC, the fusiform gyrus, the inferior temporal and inferior frontal gyrus, the amygdala, insula, putamen, caudate nucleus, the ACC, but also in hippocampal and thalamic regions. These results partially match the results in healthy subjects, when asking for differential responses regarding the hetero- or homosexual orientation. In healthy hetero- and homosexual subjects the hypothalamus, posterior parietal areas, the premotor cortex, and the ventral striatum respond differentially to the preferred stimulus.
These brain structures are linked with the cognitive, motivational and the autonomic component. In pedophiles several brain regions, which are mainly linked with the cognitive, emotional and autonomic component of sexual arousal seem to respond differentially to child and adult stimuli, (see table 4). Recently, it has been shown, that already the presentation of child's and adult's faces elicit differential brain activation patterns in healthy subjects and pedophiles (Ponseti et al., 2014).
At the same time, it is questionable if it is plausible to compare these activation patterns in pedophiles to the results of studies examining sexual preference in healthy subjects. The latter asks for differences regarding the gender of the stimuli - e.g. the preference for women or men. In contrast, studies examining pedophilic sexual preference ask for differences with respect to the age of the presented stimulus, e.g. child or adult. In general, each stimulus can serve as a sexually preferred stimulus. One approach to distinguish both effects was presented by Habermeyer et al. (2013) (see table 1). In their study an analysis of variance with the factors age and gender of the stimulus was applied. According to the results the factor gender explained variance in various brain structures, whereas the factor age only explained variance in the superior frontal gyrus. Considering the ongoing debate on defining pedophilia as a sexual orientation (Schmidt et al., 2013; Seto, 2012), both factors (gender and age of the presented stimuli) should be considered and analyzed in imaging studies.
Table 4: Results of the brain
imaging studies examining pedophilic interest for the comparison of the
sexual preferred stimuli and the non-preferred stimuli in the pedophile
subjects (or similar appropriate comparisons). The activated brain regions
are arranged according to the four-component model by Stoleru et al. 2012 |
| |
Four-componet
model |
Excitatory
components |
Inhibitory
component |
Additional
brain regions not assigned to the four-component model |
| |
Component |
Cognitive |
Emotional |
Motivational |
Autonomic |
|
|
| |
Brain Region |
Attention: IPL, SPL
Appraisal: right lateral OFC, ITG
Motor imagery: vPM, SMA, CB, IPL |
Somatosensory cortex (SI, SII)
Amygdala,
Posterior Insula |
ACC, claustrum, posterior parietal cortex, hypothalamus,
substantia nigra, ventral striatum |
ACC, anterior Insula, putamen, hypothalamus |
Inhibition and devaluation:
medial and left lateral OFC, lat. temporal Cortex
Inhibition of motor reaction:
caudate nucleus, cACC |
|
| Study |
comparison |
|
|
|
|
|
|
| Dressing et al. 2001 |
Boy vs. woman |
Right OFC ,
Fusiform gyrus (ITG) |
|
ACC
(basal ganglia) |
ACC |
|
Occipital cortex,
brain stem
prefrontal cortex |
| Cohen et al. 2002 |
No such contrast |
|
|
|
|
|
|
| Wiebking et al. 2006 |
No such contrast |
|
|
|
|
|
|
| Walter et al. 2007 |
No such contrast |
|
|
|
|
|
|
| Sartorius et al.2008 |
Children vs. adults |
|
Amygdala |
|
|
|
|
| Schiffer et al. 2008 |
Girl vs. woman |
|
Insula |
|
Insula |
|
Frontal lobe: DLPAFC, Thalamus, Hippocampus,
inferior, middle occipital gyrus |
| Schiffer et al. 2008 |
Boy vs. man |
Inferior temporal gyrus |
Insula, Amygdala |
|
Insula |
|
Inferior and middle frontal gyrus,
Parahippocampal gyrus, precuneus,
middle temporal gyrus,
middle occipital gyrus |
| Poeppl et al. 2011 |
Children vs. adult |
Fusiform gyrus, Cerebellum,
inferior frontal gyrus (orbital) |
Insula |
|
Insula, putamen |
Superior, middle temporal gyrus,
inferior frontal gyrus (orbital) |
Inferior, middle, medial frontal gyrus,
hippocampus, parahippocampal gyrus, precuneus,
thalamus,
midbrain,
cuneus,
middle, inferior occipital gyrus, |
| Ponseti et al. 2012 |
No such contrast |
|
|
|
|
|
|
| Habermeyer et al. 2013 |
Girl vs. woman |
Right lat. OFC |
|
|
|
|
|
| Ponseti et a. 2014 |
child’s faces vs. adult’s faces |
|
|
|
Putamen |
Caudate nucleus |
Sulcus calacrinus,Inferior occipital gryus, fusiform
gyrus, ventrolateral prefrontal cortex |
Abbreviations:
AM: Amygdala, ACC: anterior cingulate cortex, CB: Cerebellum, IPL: inferior
parietal lobule, OFC: orbitofrontal cortex, SMA: supplementary motor area,
SPL: superior parietal lobule, TC: temporal cortex, vPM: ventral premotor
cortex, , VLPFC: ventral lateral prefrontal cortex, DLPFC, dorsal lateral
prefrontal cortex, FPPFC: frontopolar prefrontal cortex
CG: Control group, EG: Experimental group |
FMRI as an assessment tool for sexual preference - a first approach
Till this day, we do not have a convenient clinical tool to assess sexual preference using brain imaging methods. A first approach to establish such a tool was published by Ponseti et al. (2012). The authors used a new automatic classification approach to differentiate between normophilic and pedophilic subjects (Soriano-Mas et al., 2007). This study will be discussed in more detail, pointing out the potential as well as the limitations of the study.
In this fMRI-study, Ponseti et al. examined 24 pedophilic outpatients and 32 healthy male controls. Each pedophilic patient openly admitted to pedophilia. According to the self-report questionnaire, seven out of the 24 pedophilic subjects belonged to the non-exclusive type. The groups were matched in terms of age and intelligence. The task required the subjects to passively watch images of an entire person or genitals, which were compared to a set of non-sexual stimuli (see table 1). Besides the already described analyses (see table 2, 3, and 4), an individual expression value was computed for each subject in order to determine the classification accuracy. This individual expression value represented the difference between the individual differential functional imaging maps (e.g. hemodynamic responses for the comparison: boys vs. men; girls vs. women) and the group mean for the same contrasts. These individual expression values were submitted to two different classification analyses. The Fishers linear discriminant classification analyses revealed the best classification results for the four classes: the hetero- and homosexual healthy subjects and the hetero- and homosexual pedophilic subjects. Only three participants were misclassified (as false negative). Out of the three misclassified pedophiles two were heterosexual and one was homosexual. All of them belonged to the non-exclusive type. For this classification analysis a specificity of 100% and a sensitivity of 88% was reached.
In summary, using the individual brain activation patterns of visual sexual stimuli, i.e. the individual differences of the group mean, Ponseti et al. were able to classify normophilic subjects and pedophilic patients correctly according to their individual sexual preference. This new approach is of great interest, because it not only shows the group differences but it could also be able to differentiate between normophilic and pedophilic sexual preference on the basis of the individual brain activation pattern of the subject. Nevertheless, several issues have to be discussed, mostly claiming for further validation studies with other subjects, e.g. denying their pedophilic preference. Another crucial limitation indicates the inclusion of exclusive and non-exclusive pedophiles in one group, as shown in the misclassification of three non-exclusive pedophilic subjects. The intelligence was only matched up using one subtest of the most common IQ-test, which could be problematic due to the well-known fact that pedophiles often show lower intelligence scores than healthy subjects. Furthermore, it is not quite clear if the presentation of child genitals leads to the sexual arousal patterns as described in the sections above, or might also cause feelings of disgust, shame or even psychological strain.
GENERAL DISCUSSION & POTENTIAL AND LIMITS OF THE ASSESSMENT OF SEXUAL PREFERENCE USING FMRI - SUGGESTIONS FOR FUTURE DEVELOPMENT
General discussion
The objective assessment of sexual preference, i.e. independent of the patient's subjective response, is of great importance in the sector of forensic psychiatry. But until now we do not have a reliable clinical tool to assess sexual preference based on brain imaging methods. With respect to the extensive research in healthy subjects regarding the neural underpinnings of sexual arousal and preference we presented an overview about the most current knowledge in healthy subjects and the few functional imaging studies in paraphilic patients.
In summary, the description of the most common experimental designs of imaging studies to assess sexual arousal and sexual preference demonstrated that passive viewing paradigms are used in the majority of studies with stimuli presentation times of several seconds. Sexually explicit stimuli are preferably compared to non-sexual stimuli. But the studies vary according to a lot of methodological characteristics. This large variability should be considered throughout the evaluation or comparison of the different results of the studies.
According to current theoretical and neurobiological models sexuality is defined in the context of theories of emotion and motivation. Based on functional neuroimaging studies a neurobiological four-component model of sexual arousal was introduced, which proposed a cognitive, an emotional, a motivational and an autonomic excitatory component as well as an inhibitory component, each interacting with one and another (Stoléru et al., 2012). A lot of studies with the focus on healthy heterosexual male subjects found a complex pattern of responsive brain areas, supporting this four-component model (see fig.1.). Nevertheless, none of these brain regions are specifically or exclusively associated with the processing of sexual stimuli, but rather, they are of great influence in several other non-sexual functions. Additionally, not all the studies, which are included in the review of Stoléru et al. (2012), found an activation of all the brain regions of the four-component model. One conceivable reason could lie in the different methodological designs and control conditions. Additionally, the type of analysis may also contribute to these differences. It was shown that the brain activations analyzed in relation to visual sexual stimuli differed from brain activation patterns in association with penile erection (Poeppl et al., 2013). Another neurobiological approach describing neurobiology of sexual arousal was presented by Georgiadis and Kringelbach (2012) using the definition of sexuality in the sense of a reward behavior. This interesting model could extend the four-component model by looking at the whole sexual response cycle.
A few studies addressed the question of the impact of sexual preference on these activation patterns. Interestingly, it appears that the presentation of the sexually preferred stimulus leads to similar patterns in hetero- and homosexual subjects. However, some brain areas respond more specifically to the preferred sexual stimulus when compared to the non-preferred stimulus. Especially the thalamus and the hypothalamus, which are connected to the autonomic and motivational aspects of sexual arousal, as well as some brain regions associated with the cognitive component, seem to respond exclusively to the preferred sexual stimulus. Despite these interesting results, the poor amount of studies and the methodological differences have to be kept in mind. Sadly, to this day, the question of whether or nor not certain brain structures respond in a specific manner depending on the individual sexual orientation, has not been answered yet.
Discussing the neural underpinnings of deviant sexual preference in paraphilic subjects one should also include the possible structural abnormalities found in paraphilic patients. Despite the comprehensive Integrated Theory of Sexual Offending (ITSO) there are only three theories which tried to describe the neurobiological basis of paraphilia based on recent findings of brain imaging studies. These theories focused on the special case of pedophilia: the frontal-dysexecutive theory, the temporal-limbic theory and the dual-dysfunctional theory which connects the former theories. Interestingly, the structural abnormalities found in paraphilic patients, partially support these theories.
Regarding functional imaging we discussed the experimental methods used in the ten brain imaging studies to assess sexual preference in pedophiles. Table 1 summarized the experimental details. Studies differ with respect to the number of included subjects, the presented experimental and control stimuli, the stimulus presentation time and also with respect to the applied paradigm. Similar to the studies of healthy subjects, in most of these studies, passive viewing designs were applied, presenting the stimuli for several seconds. In contrast to studies with healthy subjects, in this case sexual non-explicit stimuli have been presented. Control conditions varied from non-sexual stimuli, to emotional control stimuli and sexual non-preferred stimuli. Most studies compared paraphilic patients with healthy subjects; only one study included a forensic control group (Poeppl et al., 2011). Furthermore, different methods and comparisons were applied to analyze the data. Despite the interesting and new results, these methodological differences might be an indication for the great variability regarding the results of these studies.
In general, the results of these ten studies demonstrate that pedophilic subjects show similar brain activations compared to healthy subjects while watching their preferred sexual stimulus. This allows the conclusion that the four-component model of sexual arousal can also be applied to pedophiles. After all, not all studies reported brain activations in each of the four components. From a methodological point of view, the use of non-explicit sexual stimuli in contrast to the use of explicit sexual stimuli in studies with healthy subjects could play a crucial role.
Up to date it is not quite clear if pedophiles respond generally stronger or weaker than normophilic subjects to their preferred stimulus. Also activation patterns of the difference between the hemodynamic responses to the preferred compared to the non-preferred sexual stimulus, do not show a clear picture. Thereby, the cognitive, autonomic and emotional component of sexual arousal might play an important role. But it is questionable if one can compare the activation patterns of a sexual preference with respect to the gender (i.e. hetero- and homosexual healthy subjects) with the activation patterns with respect to age (i.e. healthy subjects and pedophilic subjects). Considering the ongoing debate on defining pedophilia as a sexual orientation (Schmidt et al., 2013; Seto, 2012), both factors (gender and age of the presented stimuli) should be considered and analyzed in imaging studies. One approach to analyze both effects differentially was presented by Habermeyer et al. (2013). They applied an analysis of variance with the factors age and gender of the stimulus and found different brain regions to be associated with both factors.
We also discussed a first approach using fMRI as a clinical tool for the assessment of sexual (deviant) preference (Ponseti et al., 2012). Using a new automatic classification it was possible to differentiate between normophilic and pedophilic subjects. This automatic classification approach seems to be a promising objective clinical tool to assess sexual deviant preference, but further research is needed.
Potential & limitations of the assessment of sexual preference using fMRI - Suggestions for future development
The application of functional brain imaging methods has significantly enhanced our knowledge about the neural correlates of sexual arousal and sexual preference. The results of functional brain imaging methods measuring sexual arousal and sexual preference deliver a new and complex picture about the neural underpinnings of those functions in healthy subjects and paraphilic patients. In contrast to clinical interviews or questionnaires, the application of functional neuroimaging methods are a promising step towards a tool to assess sexual preference without a (verbal) subjective response of the patient. But only one study demonstrated this approach using an automatic classification algorithm. For this reason, further research is needed before functional imaging can be used as a reliable clinical tool.
Recently, four case studies showed that fMRI can also detect changes in brain activation patterns in response to visual sexual stimuli probably evoked by antiandrogen treatment (Habermeyer et al., 2012; Jordan, Fromberger, Laubinger, Dechent, & Müller, 2014; Moulier et al., 2012; Schiffer, Gizewski, & Krüger, 2009). This approach demonstrates the potential of neuroimaging methods in evaluating therapeutic effects in forensic psychiatry. Up to now, this is only demonstrated for antiandrogen therapy based on a few case studies. But it seems also possible to depict psychotherapeutic changes as it has already been done in regard to other psychiatric disorders (e.g. (Hoflich, Baldinger, Savli, Lanzenberger, & Kasper, 2012)).
Combining the eye tracking method simultaneously with functional imaging could also give a more detailed insight in the underlying neurofunctional processes of a deviant sexual interest and at the same time increase the diagnostic accuracy. But until now, no such study has been published. In summary, functional neuroimaging techniques provide much more potential for a reliable clinical application regarding paraphilia than it has been shown until now.
Besides the development of the recently brought up approaches to assess sexual preference, we propose the improvement of the experimental designs. According to us, it is indispensable to include a forensic control group in order to accomplish a better group matching. Furthermore, an active task capturing the subject's attention could contribute to a lower individual variability between the subjects (Wieser, Methfessel, Jordan, Fromberger, & Müller, 2014). Another stimulation design to avoid possible manipulations by the subjects seems to be the subliminal presentation of visual sexual stimuli. Subliminal stimuli are presented with time duration of 50 ms at the most. The threshold of 50 ms ensures that the stimuli are in most instances not consciously perceivable for the subjects. This, in turn, decreases the possibility to manipulate the subjective reaction. It has been shown that the subliminal presentation of visual sexual stimuli can elicit hemodynamic responses in brain areas which are linked with the four components of sexual arousal (Gillath & Canterberry, 2012; Hofter, Fromberger, Jordan, & Müller, 2014). Due to ethical aspects we recommend to use non-explicit sexual stimuli. As demonstrated in this review, similar to explicit sexual stimuli, non-explicit sexual stimuli could evoke hemodynamic responses in brain regions which are equally linked with the four components of sexual arousal. If the technical adjustment is possible, additional psychophysiological measures, e.g. skin conductance or PPG could enhance the information about the processing of sexual stimuli.
Even if we are convinced that in the future the application of functional brain imaging can be a useful tool to assess sexual preference it should always be used in combination with other diagnostic instruments, i.e. clinical interview, questionnaires, and direct or indirect psychophysiological methods. A potential fMRI-tool could reveal additional information about the sexual preference of the subject, which is more or less independent of the subjective answer of the subject. The combination of the different methods could enhance the diagnostic accuracy.
Acknowledgments
We thank Sarah Jung for careful linguistic revision of the manuscript.
Note
This article is a modified and updated version of the chapter "The measurement of sexual arousal and sexual preference in healthy and paraphilic subjects using functional imaging" published in: The Wiley-Blackwell Handbook on the Assessment, Treatment and Theories of Sexual Offending, Volume: Assessment. Eds. Leam A. Craig and Martin Rettenberger, John Wiley & Sons, 2014.
References- Bancroft, J. (2009). Models of human sexuality: the role of
theory. In J. Bancroft (Ed.), Human sexuality and its problems (3thrd
ed., pp. 5-19). Edinburgh: Churchill Livingstone.Bancroft, J., Graham,
C. A., Janssen, E., & Sanders, S. A. (2009). The dual control model:
current status and future directions. Journal of Sex Research, 46(2),
121-142.
- Bancroft, J., & Janssen, E. (2000). The dual
control model of male sexual response: a theoretical approach to
centrally mediated erectile dysfunction. Neuroscience and Biobehavioral
Reviews, 24(5), 571-579.
- Beech, A. R., Kalmus, E., Tipper, S.
P., Baudouin, J. Y., Flak, V., & Humphreys, G. W. (2008). Children
induce an enhanced attentional blink in child molesters. Psychological
Assessment, 20(4), 397-402. doi: 10.1037/a0013587
- Blanchard, R.,
Klassen, P., Dickey, R., Kuban, M. E., & Blak, T. (2001).
Sensitivity and specificity of the phallometric test for pedophilia in
nonadmitting sex offenders. Psychological Assessment, 13(1), 118-126.
- Brown,
A. S., Gray, N. S., & Snowden, R. J. (2009). Implicit measurement
of sexual associations in child sex abusers. Sexual Abuse: A Journal of
Research and Treatment, 21(2), 166-180.
- Burns, J. M., &
Swerdlow, R. H. (2003). Right orbitofrontal tumor with pedophilia
symptom and constructional apraxia sign. Archives of Neurology, 60(3),
437-440.
- Cantor, J. M., Kabani, N., Christensen, B. K.,
Zipursky, R. B., Barbaree, H. E., Dickey, R., Klassen, P. E., Mikulis,
D. J., Kuban, M. E., Blak, T., Richards, B. A., Hanratty, M. K., &
Blanchard, R. (2008). Cerebral white matter deficiencies in pedophilic
men. Journal of Psychiatric Research, 42(3), 167-183.
- Cohen, L.
J., Nikiforov, K., Gans, S., Poznansky, O., McGeoch, P., Weaver, C.,
King, E. G., Cullen, K., & Galynker, I. (2002). Heterosexual male
perpetrators of childhood sexual abuse: a preliminary neuropsychiatric
model. Psychiatric Quarterly, 73(4), 313-336.
- Dressing, H.,
Obergriesser, T., Tost, H., Kaumeier, S., Ruf, M., & Braus, D. F.
(2001). Homosexuelle Pädophilie und funktionelle Netzwerke - eine
fMRI-Fallstudie. [Homosexual pedophilia and functional networks - An
fMRI case report and literature review]. Fortschritte der
Neurologie-Psychiatrie, 69(11), 539-544.
- Everaerd, W. (1988). Commentary on sex research: Sex as an emotion. Journal of Psychology & Human Sexuality, 1(2), 3-15.
- Freund,
K., & Blanchard, R. (1989). Phallometric diagnosis of pedophilia.
Journal of Consulting and Clinical Psychology, 57(1), 100-105.
- Freund,
K., Diamant, J., & Pinkava, V. (1958). On the validity and
reliability of the phalloplethysmographic (Php) diagnosis of some sexual
deviations. Review of Czechoslovak Medicine, 4(2), 145-151.
- Fromberger,
P. (2010). Funktionelle Magnetresonanztomographie und
Blickregistrierung bei sexueller Erregung: Neue Perspektiven für die
Grundlagenforschung bei pädosexuellen Straftätern? In J.L. Müller (Ed.),
Neurobiologie forensisch-relevanter Störungen (pp. 440-452). Stuttgart:
Kohlhammer.
- Fromberger, P., Jordan, K., Steinkrauss, H., von
Herder, J., Stolpmann, G., Kröner-Herwig, B., & Müller, J. L.
(2013). Eye movements in pedophiles: Automatic and controlled
attentional processes while viewing prepubescent stimuli. Journal of
Abnormal Psychology, 122(2), 587-599. doi: 10.1037/a0030659
- Fromberger,
P., Jordan, K., Steinkrauss, H., von Herder, J., Witzel, J., Stolpmann,
G., Kröner-Herwig, B., & Müller, J. L. (2012). Diagnostic accuracy
of eye movements in assessing pedophilia. The Journal of Sexual
Medicine, 9(7), 1868-1882. doi: 10.1111/j.1743-6109.2012.02754.x
- Fromberger,
P., Krippl, M., Stolpmann, G., & Müller, J. (2007). Neurobiologie
der pädophilen Störung - eine methodenkritische Darstellung bisheriger
Forschungsergebnisse [Neurobiology of pedophilia - a critical review of
present findings and paradigms]. Forensische Psychiatrie, Psychologie,
Kriminologie, 1(4), 249-258.
- Fromberger, P., Stolpmann, G.,
Jordan, K., & Müller, J. L. (2009). Neurobiologische Forschung bei
Pädophilie - Ergebnisse und deren Konsequenzen für die Diagnostik
pädosexueller Straftäter [Neurobiological research on pedophilia -
results and their consequences for the assessment of pedophilic
offenders]. Zeitschrift fuer Neuropsychologie, 20(3), 193-205.
- Georgiadis,
J. R., & Kringelbach, M. L. (2012). The human sexual response
cycle: Brain imaging evidence linking sex to other pleasures. Progress
in Neurobiology, 98(1), 49-81. doi: 10.1016/j.pneurobio.2012.05.004
- Gillath,
O., & Canterberry, M. (2012). Neural correlates of exposure to
subliminal and supraliminal sexual cues. Social Cognitive and Affective
Neuroscience, 7(8), 924-936. doi: 10.1093/scan/nsr065
- Glasgow,
D. V. (2009). Affinity: The development of a self-report assessment of
paedophile sexual interest incorporating a viewing time validity
measure. In D. Thornton & D. R. Laws (Eds.), Cognitive Approaches to
the Assessment of Sexual Interest in Sexual Offenders (pp. 59-84).
Chichester: Wiley-Blackwell.
- Graber, B., Hartmann, K., Coffman,
J. A., Huey, C. J., & Golden, C. J. (1982). Brain damage among
mentally disordered sex offenders. Journal of Forensic Sciences, 27(1),
125-134.
- Habermeyer, B., Esposito, F., Handel, N., Lemoine, P.,
Klarhofer, M., Mager, R., Dittmann, V., Seifritz, E., & Graf, M.
(2013). Immediate processing of erotic stimuli in paedophilia and
controls: a case control study. BMC Psychiatry, 13(1), 88.
- Habermeyer,
B., Händel, N., Lemoine, P., Klarhöfer, M., Seifritz, E., Dittmann, V.,
& Graf, M. (2012). LH-RH agonists modulate amygdala response to
visual sexual stimulation: A single case fMRI study in pedophilia.
Neurocase, 18(6), 489-495. doi: 10.1080/13554794.2011.627346
- Hanson,
R. K., & Morton-Bourgon, K. E. (2005). The characteristics of
persistent sexual offenders: A meta-analysis of recidivism studies.
Journal of Consulting and Clinical Psychology, 73(6), 1154-1163.
- Hoflich,
A., Baldinger, P., Savli, M., Lanzenberger, R., & Kasper, S.
(2012). Imaging treatment effects in depression. Reviews in the
Neurosciences, 23(3), 227-252. doi: 10.1515/revneuro-2012-0038
- Hofter,
C., Fromberger, P., Jordan, K., & Müller, J. L. (2014). Die
Verarbeitung subliminal dargebotener visueller sexueller Reize - Eine
fMRT-Studie mit gesunden heterosexuellen Männern. In J. L. M. Michael
Rösler, Peer Briken, Petra Retz-Junginger, Wolfgang Retz, Florence
Philipp-Wiegemann (Ed.), EFPPP Jahrbuch 2014. Empirische Forschung in
der forensischen Psychiatrie, Psychologie und Psychotherapie. Berlin:
Medizinisch Wissenschaftliche Verlagsgesellschaft.
- Janssen, E.,
Everaerd, W., Spiering, M., & Janssen, J. (2000). Automatic
processes and the appraisal of sexual stimuli: Toward an information
processing model of sexual arousal. Journal of Sex Research, 37(1),
8-23.
- Jordan, K., Fromberger, P., Laubinger, H., Dechent, P.,
& Müller, J. L. (2014). Changed processing of visual sexual stimuli
under GnRH-therapy - a single case study in pedophilia using eye
tracking and fMRI. BMC Psychiatry, 14(1), 142.
- Jordan, K.,
Fromberger, P., & Müller, J. L. (2013). Neurobiologie der Sexualität
und sexueller Störungen. In P. Briken & M. Berner Michael (Eds.),
Praxisbuch Sexuelle Störungen. Stuttgart: Thieme Verlag.
- Jordan,
K., Fromberger, P., Stolpmann, G., & Muller, J. L. (2011a). The
role of testosterone in sexuality and paraphilia-a neurobiological
approach. Part I: testosterone and sexuality. Journal of Sexual
Medicine, 8(11), 2993-3007. doi: 10.1111/j.1743-6109.2011.02394.x
- Jordan,
K., Fromberger, P., Stolpmann, G., & Muller, J. L. (2011b). The
Role of Testosterone in Sexuality and Paraphilia-A Neurobiological
Approach. Part II: Testosterone and Paraphilia. Journal of Sexual
Medicine, 8(11), 3008-3029. doi: 10.1111/j.1743-6109.2011.02393.x
- Joyal,
C. C., Black, D. N., & Dassylva, B. (2007). The neuropsychology and
neurology of sexual deviance: A review and pilot study. Sexual Abuse: A
Journal of Research and Treatment, 19(2), 155-173.
- Kagerer,
S., Klucken, T., Wehrum, S., Zimmermann, M., Schienle, A., Walter, B.,
Vaitl, D., & Stark, R. (2011). Neural activation toward erotic
stimuli in homosexual and heterosexual males. Journal of Sexual
Medicine, 8(11), 3132-3143. doi: 10.1111/j.1743-6109.2011.02449.x
- Kaplan, H. (1979). Disorders of sexual desire and other new concepts and techniques in sex therapy. New York: Brunner/Mazel.
- Kaplan,
M. S., & Krueger, R. B. (2010). Diagnosis, assessment, and
treatment of hypersexuality. Journal of Sex Research, 47(2), 181-198.
- Kranz, F., & Ishai, A. (2006). Face perception Is modulated by sexual preference. Current Biology, 16(1), 63-68.
- Krüger,
T. H. C., & Schiffer, B. (2011). Neurocognitive and personality
factors in homo- and heterosexual pedophiles and controls. Journal of
Sexual Medicine, 8(6), 1650-1659.
- Lang, P. J., Bradley, M. B.,
& Cuthbert, B. N. (2001). International Affective Picture System,
2001. NIMH Center for the Study of Emotion and Attention: University of
Florida, Gainesville, FL
- Laws, D. R., & Gress, C. L. Z.
(2004). Seeing things differently: The viewing time alternative to
penile plethysmography. Legal and Criminological Psychology, 9(2),
183-196.
- Marshall, W. L. (2014). Phallometric assessments of
sexual interests: an update. Current Psychiatry Reports, 16(428), 1-7.
doi: 10.1007/s11920-013-0428-6
- Masters, W. H., & Johnson, V. E. (1966). Human sexual response. Boston: Little, Brown & Co.
- Mohnke,
S., Muller, S., Amelung, T., Kruger, T. H., Ponseti, J., Schiffer, B.,
Walter, M., Beier, K. M., & Walter, H. (2014). Brain alterations in
paedophilia: A critical review. [Review]. Prog Neurobiol. doi:
10.1016/j.pneurobio.2014.07.005
- Mokros, A., Butz, M., Dombert,
B., Santtila, P., Bäuml, K.-H., & Osterheider, M. (2011). Judgment
of age and attractiveness in a paired comparison task: Testing a picture
set developed for diagnosing paedophilia. Legal and Criminological
Psychology, 16(2), 323-334. doi: 10.1348/135532510x514104
- Mokros,
A., Dombert, B., Osterheider, M., Zappala , A., & Santtila, P.
(2010). Assessment of pedophilic sexual interest with an attentional
choice reaction time task. Archives of Sexual Behavior, 39(5),
1081-1090.
- Molenberghs, P., Cunnington, R., & Mattingley,
J. B. (2012). Brain regions with mirror properties: A meta-analysis of
125 human fMRI studies. Neuroscience and Biobehavioral Reviews, 36(1),
341-349. doi: 10.1016/j.neubiorev.2011.07.004
- Moulier, V.,
Fonteille, V., Pelegrini-Issac, M., Cordier, B., Baron-Laforet, S.,
Boriasse, E., Durand, E., & Stoleru, S. (2012). A pilot study of the
effects of gonadotropin-releasing hormone agonist therapy on brain
activation pattern in a man with pedophilia. International Journal of
Offender Therapy and Comparative Criminology, 56(1), 50-60. doi:
10.1177/0306624X10392191
- O'Donohue, W., Regev, L. G., &
Hagstrom, A. (2000). Problems with the DSM-IV diagnosis of pedophilia.
Sexual Abuse: A Journal of Research and Treatment, 12(2), 95-105.
- Otti,
A., Gündel, H., Wohlschläger, A., Zimmer, C., Sorg, C., &
Noll-Hussong, M. (2012). „Default-mode"-Netzwerk des Gehirns. Der
Nervenarzt, 83(1), 16-24. doi: 10.1007/s00115-011-3307-6
- Pacific
Psychological Assessment Corporation. (2004). The NRP (Not Real People)
stimulus set for the assessment of sexual interest. Victoria, BC:
Pacific Psychological Assessment Corporation.
- Paul, T.,
Schiffer, B., Zwarg, T., Krüger, T. H., Karama, S., Schedlowski, M.,
Forsting, M., & Gizewski, E. R. (2008). Brain response to visual
sexual stimuli in heterosexual and homosexual males. Human Brain
Mapping, 29(6), 726-735.
- Pennington, B. F. (2002). The development of psychopathology: Nature and nurture. New York: Guilford Press.
- Poeppl,
T. B., Langguth, B., Laird, A. R., & Eickhoff, S. B. (2013). The
functional neuroanatomy of male psychosexual and physiosexual arousal: A
quantitative meta-analysis. Human Brain Mapping. doi: 10.1002/hbm.22262
- Poeppl,
T. B., Nitschke, J., Dombert, B., Santtila, P., Greenlee, M. W.,
Osterheider, M., & Mokros, A. (2011). Functional cortical and
subcortical abnormalities in pedophilia: A combined study using a choice
reaction time task and fMRI. Journal of Sexual Medicine, 8(6),
1660-1674.
- Polisois-Keating, A., & Joyal, C. (2013).
Functional Neuroimaging of Sexual Arousal: A Preliminary Meta-Analysis
Comparing Pedophilic to Non-Pedophilic Men. Archives of Sexual Behavior,
42(7), 1111-1113. doi: 10.1007/s10508-013-0198-6
- Ponseti, J.,
Bosinski, H. A., Wolff, S., Peller, M., Jansen, O., Mehdorn, H. M.,
Büchel, C., & Siebner, H. R. (2006). A functional endophenotype for
sexual orientation in humans. NeuroImage, 33(3), 825-833.
- Ponseti,
J., Granert, O., Jansen, O., Wolff, S., Beier, K., Neutze, J., Deuschl,
G., Mehdorn, H., Siebner, H., & Bosinski, H. (2012). Assessment of
pedophilia using hemodynamic brain response to sexual stimuli. Archives
of General Psychiatry, 69(2), 187-194. doi:
10.1001/archgenpsychiatry.2011.130
- Ponseti, J., Granert, O., van
Eimeren, T., Jansen, O., Wolff, S., Beier, K., Deuschl, G., Bosinski,
H., & Siebner, H. (2014). Human face processing is tuned to sexual
age preferences. Biology Letters, 10(5). doi: 10.1098/rsbl.2014.0200
- Raichle,
M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A.,
& Shulman, G. L. (2001). A default mode of brain function.
Proceedings of the National Academy of Sciences of the United States of
America, 98(2), 676-682. doi: 10.1073/pnas.98.2.676
- Rizzolatti, G., & Craighero, L. (2004). The Mirror-Neuron System. Annual Review of Neuroscience, 27(1), 169-192.
- Rosen,
R. C., & Beck, J. G. (1988). Patterns of sexual arousal:
Psychophysiological processes and clinical applications: : Guilford
Press.
- Sachsenmaier, S. J., & Gress, C. L. Z. (2009). The
abel assessment for sexual interests - 2: A critical review. In D.
Thornton & D. R. Laws (Eds.), Cognitive Approaches to the Assessment
of Sexual Interest in Sexual Offenders (pp. 31-57). Chichester:
Wiley-Blackwell.
- Sartorius, A., Ruf, M., Kief, C., Demirakca,
T., Bailer, J., Ende, G., Henn, F. A., Meyer-Lindenberg, A., &
Dressing, H. (2008). Abnormal amygdala activation profile in pedophilia.
European Archives of Psychiatry and Clinical Neuroscience, 258(5),
271-277.
- Schiffer, B., Gizewski, E., & Krüger, T. (2009).
Reduced neuronal responsiveness to visual sexual stimuli in a pedophile
treated with a long-acting LH-RH agonist. Journal of Sexual Medicine,
6(3), 892-894.
- Schiffer, B., Krüger, T., Paul, T., de Greiff,
A., Forsting, M., Leygraf, N., Schedlowski, M., & Gizewski, E.
(2008). Brain response to visual sexual stimuli in homosexual
pedophiles. Journal of Psychiatry & Neuroscience, 33(1), 23-33.
- Schiffer,
B., Paul, T., Gizewski, E., Forsting, M., Leygraf, N., Schedlowski, M.,
& Krüger, T. H. C. (2008). Functional brain correlates of
heterosexual paedophilia. NeuroImage, 41(1), 80-91.
- Schiffer,
B., Peschel, T., Paul, T., Gizewski, E., Forsting, M., Leygraf, N.,
Schedlowski, M., & Krüger, T. H. (2007). Structural brain
abnormalities in the frontostriatal system and cerebellum in pedophilia.
Journal of Psychiatric Research, 41(9), 753-762.
- Schmidt, A.
F., Mokros, A., & Banse, R. (2013). Is pedophilic sexual preference
continuous? A taxometric analysis based on direct and indirect measures.
Psychological Assessment. doi: 10.1037/a0033326
- Seto, M. (2012). Is pedophilia a sexual orientation? Archives of Sexual Behavior, 1-6. doi: 10.1007/s10508-011-9882-6
- Smith,
P., & Waterman, M. (2004). Processing bias for sexual material: the
emotional stroop and sexual offenders. Sexual Abuse: A Journal of
Research and Treatment, 16(2), 163-171.
- Soriano-Mas, C., Pujol,
J., Alonso, P., Cardoner, N., Menchón, J. M., Harrison, B. J., Deus,
J., Vallejo, J., & Gaser, C. (2007). Identifying patients with
obsessive-compulsive disorder using whole-brain anatomy. NeuroImage,
35(3), 1028-1037.
- Spiering, M., & Everaerd, W. (2007). The
sexual unconscious. In E. Janssen (Ed.), The Psychophysiology of Sex
(pp. 166-183). Bloomington, Indianapolis: Indiana University Press.
- Stoléru,
S., Fonteille, V., Cornélis, C., Joyal, C., & Moulier, V. (2012).
Functional neuroimaging studies of sexual arousal and orgasm in healthy
men and women: A review and meta-analysis. Neuroscience and
Biobehavioral Reviews, 36(6), 1481-1509. doi:
10.1016/j.neubiorev.2012.03.006
- Stoléru, S., Gregoire, M. C.,
Gerard, D., Decety, J., Lafarge, E., Cinotti, L., Lavenne, F., Le Bars,
D., Vernet-Maury, E., Rada, H., Collet, C., Mazoyer, B., Forest, M. G.,
Magnin, F., Spira, A., & Comar, D. (1999). Neuroanatomical
correlates of visually evoked sexual arousal in human males. Archives of
Sexual Behavior, 28(1), 1-21.
- Sundaram, T., Jeong, G. W., Kim,
T. H., Kim, G. W., Baek, H. S., & Kang, H. K. (2010). Time-course
analysis of the neuroanatomical correlates of sexual arousal evoked by
erotic video stimuli in healthy males. Korean Journal of Radiology,
11(3), 278-285. doi: 10.3348/kjr.2010.11.3.278
- Tanner, J. M. (1973). Growing up. Scientific American, 229(3), 34-43.
- Thornton,
D., & Laws, D. R. (2009). Cognitive Approaches to the Assessment of
Sexual Interest in Sexual Offenders. West Sussex, Oxford, Malden:
Wiley-Blackwell.
- Walter, M., Witzel, J., Wiebking, C., Gubka,
U., Rotte, M., Schiltz, K., Bermpohl, F., Tempelmann, C., Bogerts, B.,
Heinze, H. J., & Northoff, G. (2007). Pedophilia is linked to
reduced activation in hypothalamus and lateral prefrontal cortex during
visual erotic stimulation. Biological Psychiatry, 62(6), 698-701.
- Ward, T., & Beech, A. (2006). An integrated theory of sexual offending. Aggression and Violent Behavior, 11(1), 44-63.
- Wiebking,
C., Witzel, J., Walter, M., Gubka, U., & Northoff, G. (2006).
Vergleich der emotionalen und sexuellen Prozessierung zwischen Gesunden
und Patienten mit einer Pädophilie- eine kombinierte Studie aus
Neuropsychologie und fMRT. Forensische Psychiatrie und Psychotherapie -
Werkstattschriften, 13 79-93.
- Wieser, K., Methfessel, I.,
Jordan, K., Fromberger, P., & Müller, J. L. (2014). Erfassung
sexueller Orientierung anhang hämodynamischer und behavioraler Korrelate
In J. L. M. Michael Rösler, Peer Briken, Petra Retz-Junginger, Wolfgang
Retz, Florence Philipp-Wiegemann (Ed.), EFPPP Jahrbuch 2014. Empirische
Forschung in der forensischen Psychiatrie, Psychologie und
Psychotherapie. Berlin: Medizinisch Wissenschaftliche
Verlagsgesellschaft.
- Wood, J. N., & Grafman, J. (2003).
Human prefrontal cortex: processing and representational perspectives.
Nature Reviews Neuroscience, 4(2), 139-147.
Author address
Kirsten Jordan, PhD
Department for Forensic Psychiatry and Psychotherapy
Georg-August-University Göttingen
Rosdorfer Weg 70,
37081 Göttingen, Germany
Kirsten.jordan@medizin.uni-goettingen.de
|